Safety & Tolerability
Weight change on CAPLYTA was similar to placebo in short-term trials1
In 4- to 6-week clinical trials, mean change in body weight from baseline at Day 28 was +3.5 lbs for CAPLYTA 42 mg and +2.9 lbs for placebo3
In a long-term study with CAPLYTA, patients saw1:
-4 lbs (average weight loss) after 6 months
-7 lbs (average weight loss) after 1 year
CAPLYTA had metabolic effects similar to placebo1
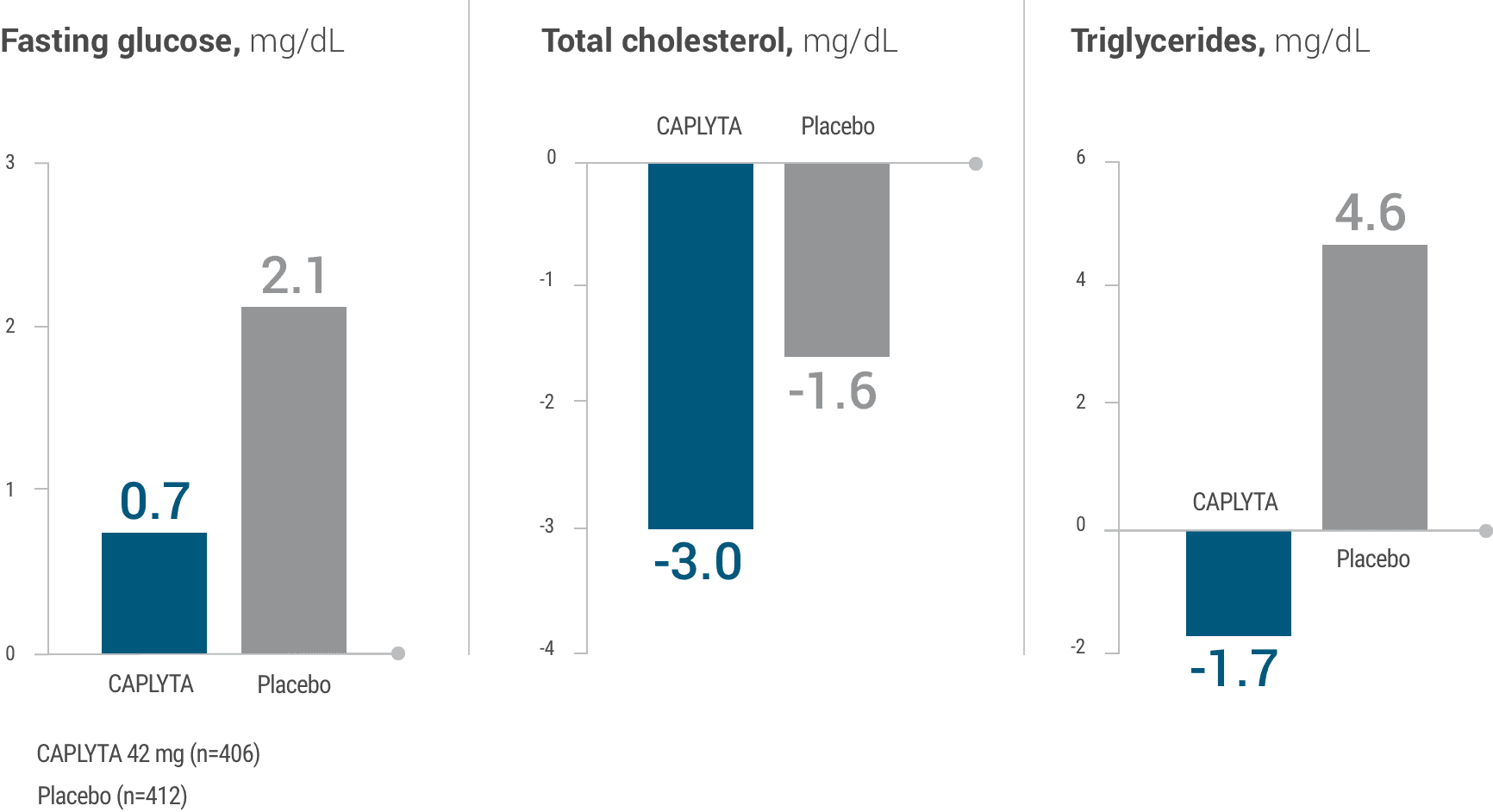
Mean change from baseline3
- CAPLYTA mean change from baseline was similar to placebo in terms of glycemic control, total cholesterol, and triglycerides1
- Data were collected in patients with acute schizophrenia over 4-6 weeks1,3
This graph depicts the mean change from baseline fasting glucose levels, total cholesterol levels, and triglycerides levels over 4-6 weeks for patients receiving CAPLYTA 42 mg or placebo.
Patients on CAPLYTA saw a 0.7-point increase in fasting glucose vs 2.1-point increase for those on placebo. Patients on CAPLYTA saw a 3.0-point reduction in total cholesterol vs 1.6-point reduction for those on placebo. Patients on CAPLYTA saw a 1.7-point reduction in triglycerides vs 4.6-point increase for those on placebo.
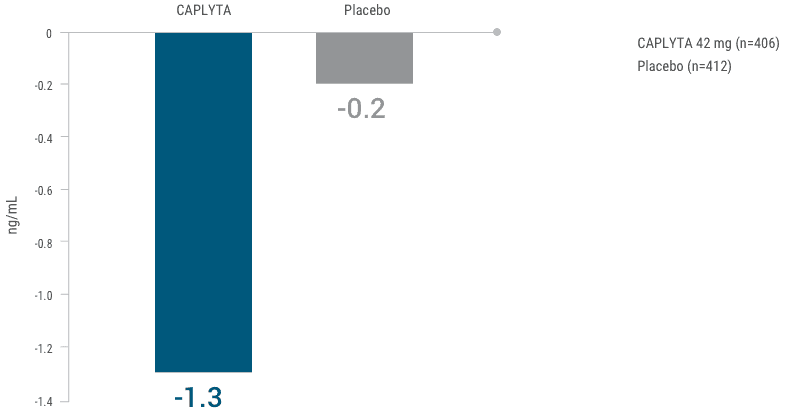
Changes in prolactin levels were similar to placebo3
Mean change in prolactin from baseline at 4 weeks3
This graph depicts the mean change in prolactin from baseline at week 4 for patients receiving CAPLYTA 42 mg or placebo.
At week 4, patients on CAPLYTA saw a 1.3-point reduction in prolactin vs 0.2-point reduction for those on placebo.
Favorable weight, metabolic, and prolactin profile was observed in a 1-year trial with CAPLYTA3
Mean change from baseline in metabolic and endocrine parameters in a long-term open-label trial3
| Blood Glucose/Insulin | Day 300* |
|---|---|
| Glucose (mg/dL) | +3.0 (n=172) |
| Insulin (mcIU/mL) | +1.0 (n=168) |
| Lipids (mg/dL) | |
|---|---|
| LDL | -7.6 (n=167) |
| HDL | -1.4 (n=172) |
| Total cholesterol | -9.6 (n=172) |
| Triglycerides | -2.5 (n=172) |
| Prolactin (ng/mL) | |
|---|---|
| Prolactin | -4.9 (n = 171) |
Changes in weight3
| Weight (lbs) | Day 175* | Day 350* |
|---|---|---|
| CAPLYTA 42 mg | -4.2 (n=328) | -7.2 (n=107) |
Data from 1-year open-label safety study. See study design below.
≥80% of patients on CAPLYTA remained normal in Key Metabolic Parameters at Day 3003‡
| Baseline Normal |
|---|
| Shifted Normal to Low | Remained Normal | Shifted Normal to High |
|---|
| Blood glucose | |||
|---|---|---|---|
| Glucose (n=172) | <1% (1/151) | 91% (138/151) | 8% (12/151) |
| Insulin (n=168) | 5% (7/134) | 83% (111/134) | 12% (16/134) |
| Lipids | |||
|---|---|---|---|
| LDL cholesterol (n=167) | 3% (4/149) | 93% (139/149) | 4% (6/149) |
| Total cholesterol (n=172) | 12% (14/120) | 80% (96/120) | 8% (10/120) |
| Triglycerides (n=172) | 4% (6/155) | 92% (142/155) | 4.5% (7/155) |
n=number of subjects with data. Baseline is defined as the last non-missing pretreatment measurement.3
Short-term (4- to 6-week) data is from a placebo-controlled study of 811 patients with schizophrenia taking doses of CAPLYTA ranging from 14 to 84 mg/day.3
Long-term data is from an open-label study of 603 stable outpatients with schizophrenia who discontinued their current antipsychotic treatment and started CAPLYTA 42 mg with no dose titration. Assessment of safety, tolerability, and efficacy were conducted at baseline and were measured at Day 8, 15, 25, and approximately every 25 days thereafter, for up to 1 year.3
The primary objective was to evaluate the safety and tolerability of CAPLYTA.3
- Metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and weight gain, have been reported with antipsychotic drugs1
- Hyperglycemia, in some cases extreme and associated with ketoacidosis, hyperosmolar coma or death, has been reported in patients treated with antipsychotics. There have been reports of hyperglycemia in patients treated with CAPLYTA1
- Assess fasting plasma glucose and lipids when initiating CAPLYTA and monitor periodically during long-term treatment1
Important Safety Information regarding metabolic effects: