
Efficacy
In 2 clinical trials, CAPLYTA demonstrated statistically superior improvement vs placebo in symptoms of schizophrenia1
Study 1. CAPLYTA demonstrated significant improvement in PANSS (Positive and Negative Syndrome Scale) total score at Day 281,2*
This study was not designed to allow for efficacy comparison of CAPLYTA and risperidone. Risperidone was included for assay sensitivity.1,2
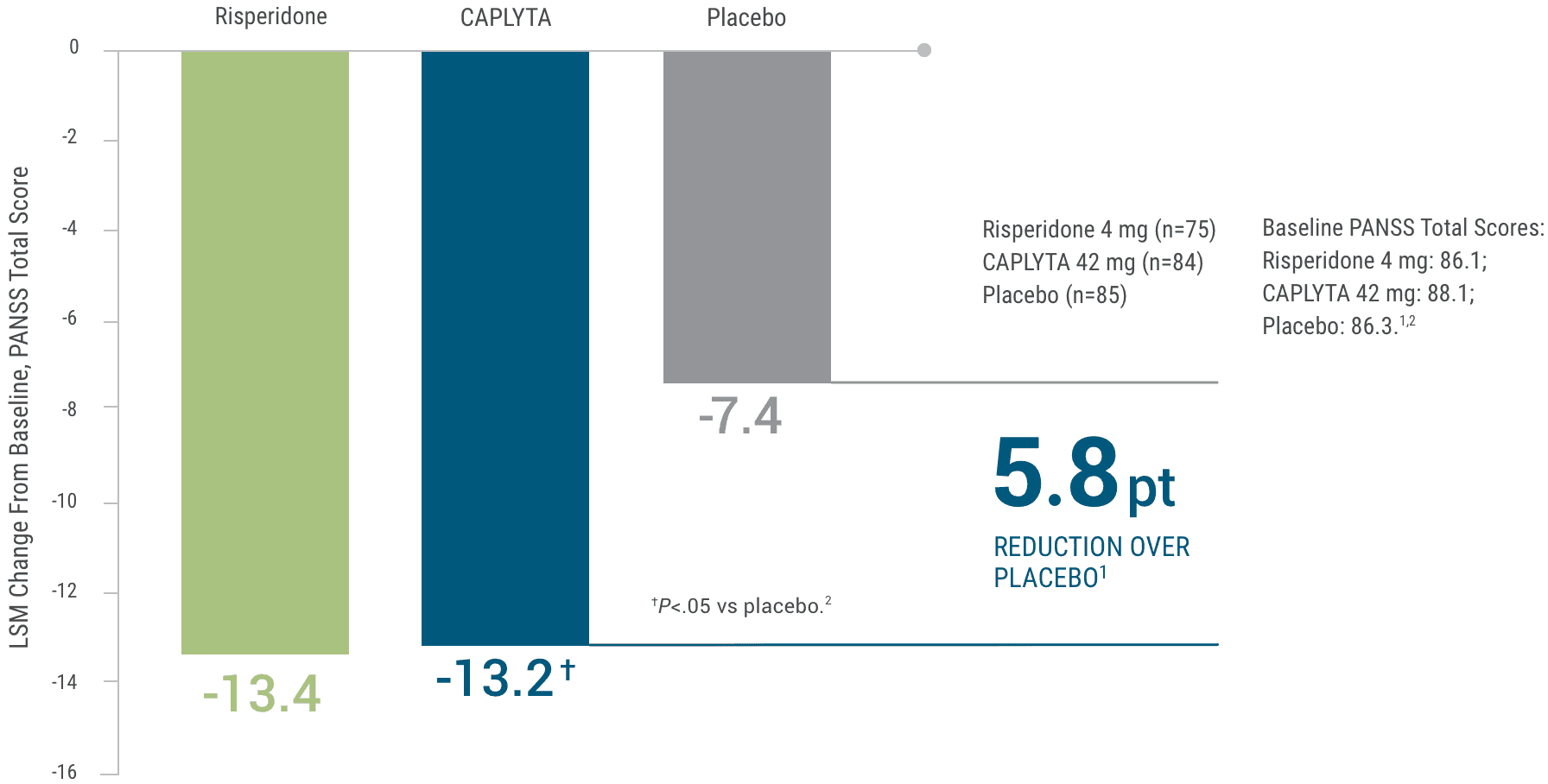
This study was not designed to allow for efficacy comparison of CAPLYTA and risperidone.
Risperidone was included for assay sensitivity.1,2
A randomized, double-blind, placebo-controlled, multicenter, inpatient clinical trial that enrolled patients with an acute exacerbation of schizophrenia. The primary endpoint was change from baseline in the PANSS total score at Day 28 compared to placebo. Patients were screened for up to a 7-day drug-free period. All treatments were dosed as oral monotherapy once daily in the morning for 4 weeks.1,2
Study 1 randomized 335 patients to either CAPLYTA 42 mg, CAPLYTA 84 mg, active comparator, or placebo in a 1:1:1:1 fashion. Patients were generally moderately to markedly ill. Median age was 42 years (range 20 to 55 years). 17% were female, 19% were Caucasian, and 78% were African American. The treatment effect in the CAPLYTA 84 mg group (vs placebo) was not statistically significant.1,3
This graph depicts the change in total PANSS score over 28 days for patients receiving risperidone 4 mg, CAPLYTA 42 mg, or placebo.
Baseline PANSS total scores were 86.1 for risperidone, 88.1 for CAPLYTA, and 86.3 for placebo. At Day 28, patients on risperidone saw a 13.4-point reduction in PANSS total score, patients on CAPLYTA saw a 13.2-point reduction in PANSS total score, and patients on placebo saw a 7.4-point reduction in PANSS total score.
CAPLYTA showed a 5.8-point reduction in PANSS total score over placebo.
Study 2. Change from baseline in PANSS total score1,4*
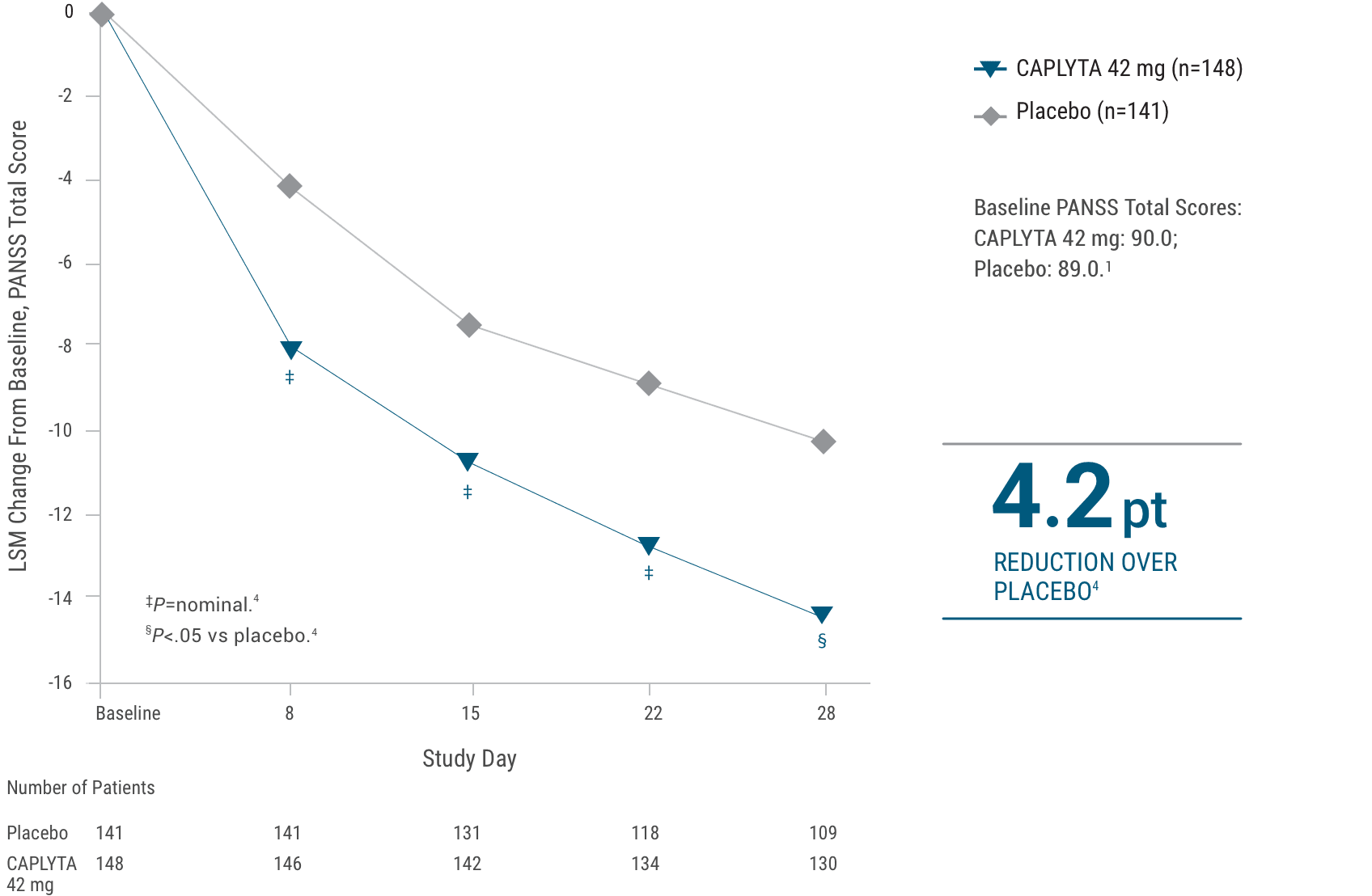
Limitation: The weekly time points prior to Day 28 were not powered for statistical comparison and are descriptive only.
A randomized, double-blind, placebo-controlled, multicenter, inpatient clinical trial that enrolled patients with an acute exacerbation of schizophrenia. The primary endpoint was change from baseline in the PANSS total score at Day 28 compared to placebo. Patients were screened for up to a 7-day drug-free period. All treatments were dosed as oral monotherapy once daily in the morning for 4 weeks.1,4
Study 2 randomized 450 patients to either CAPLYTA 28 mg, CAPLYTA 42 mg, or placebo in a 1:1:1 fashion. Patients were generally moderately to markedly ill. Median age was 44 years (range 19 to 60 years); 23% were female, 26% were Caucasian, and 66% were African American. The treatment effect in the CAPLYTA 28 mg group (vs placebo) was not statistically significant.1,3
This graph depicts the change from baseline in PANSS total score over 28 days for patients receiving CAPLYTA 42 mg or placebo.
Baseline PANSS total scores were 90.0 for CAPLYTA, and 89.0 for placebo. At Day 28, CAPLYTA showed a 4.2-point reduction in baseline PANSS total score over placebo.
CAPLYTA demonstrated significant improvement
in Clinical Global Impression-Severity (CGI-S) score3||
CGI-S was a secondary endpoint3
Patients on CAPLYTA 42 mg saw a 0.8 improvement in CGI-S score vs 0.5 on placebo (P<.05). Baseline CGI-S scores (mean) were 4.8 for CAPLYTA 42 mg and placebo.3
Frequently asked questions about efficacy
CAPLYTA was approved for the treatment of schizophrenia in adults in December 2019.1
In Study 1, risperidone was an active comparator and included for assay sensitivity.2 The study was not designed to allow for efficacy comparison of CAPLYTA and risperidone.1
In Study 2, the PANSS total score for CAPLYTA 42 mg was seen to separate from placebo as early as 8 days (first time point measured in both studies)1; however, these trials were not designed to assess early onset of effect and separation until Day 28, the primary endpoint of both studies.
CAPLYTA is indicated for the treatment of schizophrenia in adults. CAPLYTA may be used at any point across the spectrum and severity of disease.1
References: 1. CAPLYTA full prescribing information. 2. Lieberman JA, Davis RE, Correll CU, et al. CAPLYTA for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
LSM=least squares mean.
The PANSS is a 30-item scale used to measure symptoms of schizophrenia. Each item is rated by a clinician on a 7-point scale. A score of 1 indicates the absence of symptoms, and a score of 7 indicates extremely severe symptoms. The PANSS total score may range from 30 to 210, with higher scores reflecting greater overall symptom severity.1
The CGI-S asks the clinician how mentally ill the patient is, which is rated on the following 7-point scale: 1=normal, not at all ill; 2=borderline ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=among the most extremely ill patients.5
